Introduction to Perovskite Chemistry
Perovskite chemistry is a rapidly growing field within materials science, focusing on a class of compounds that share a specific crystal structure known as the perovskite structure. These materials have garnered significant attention due to their unique and versatile properties, which make them highly valuable for a wide range of technological applications.
Definition and Importance of Perovskites in Materials Science
Definition:
Perovskites are a class of materials that crystallize in a specific structure, generally described by the formula ABX₃. In this structure:
- A is a larger cation, typically an alkali metal or rare earth element.
- B is a smaller cation, often a transition metal.
- X is an anion, usually oxygen or a halogen.
The perovskite structure is characterized by a three-dimensional network of corner-sharing BX₆ octahedra, with the A cations occupying the interstitial sites. This structure can tolerate a wide range of substitutions and distortions, leading to a vast array of materials with diverse properties.
Importance:
Perovskites are important in materials science for several reasons:
- Structural Flexibility: The perovskite structure can accommodate a wide variety of elements, allowing for the tuning of electronic, magnetic, and optical properties.
- Exceptional Properties: Many perovskites exhibit remarkable properties such as high conductivity, ferroelectricity, superconductivity, and photovoltaic efficiency.
- Versatility: Perovskites can be used in a multitude of applications, including solar cells, LEDs, sensors, and catalysts.
Role of Perovskite Chemistry in Modern Technology
Perovskite chemistry plays a crucial role in modern technology, particularly in the development of new materials for energy, electronics, and environmental applications. Some key areas include:
- Photovoltaics:
- Perovskite Solar Cells: Perovskite materials, particularly organic-inorganic halide perovskites, have revolutionized the field of photovoltaics. They offer high power conversion efficiencies, low production costs, and the potential for flexible, lightweight solar panels.
- Tandem Solar Cells: Perovskites are used in tandem with silicon or other materials to create multi-junction solar cells that can capture a broader spectrum of sunlight, further enhancing efficiency.
- Light-Emitting Diodes (LEDs):
- Perovskite LEDs (PeLEDs): Perovskites are being explored for use in next-generation LEDs due to their high color purity and tunable emission wavelengths. They hold promise for displays, lighting, and even lasing applications.
- Electronics and Optoelectronics:
- Field-Effect Transistors (FETs): Perovskites are being investigated for use in FETs due to their high charge carrier mobility and ease of processing.
- Photodetectors: Perovskites are used in photodetectors for their high sensitivity and fast response times, making them suitable for imaging and communication technologies.
- Energy Storage:
- Batteries: Perovskite materials are being explored for use in solid-state batteries and other energy storage devices due to their high ionic conductivity and stability.
- Supercapacitors: Perovskites are also being investigated for use in supercapacitors, which require materials with high surface area and conductivity.
- Catalysis:
- Water Splitting: Perovskites are used as catalysts for water splitting to produce hydrogen, a clean and renewable energy source.
- Carbon Dioxide Reduction: Perovskites are being studied for their ability to catalyze the reduction of CO₂ into useful fuels and chemicals.
- Sensors:
- Gas Sensors: Perovskites are used in gas sensors due to their sensitivity to various gases and their ability to operate at high temperatures.
- Biosensors: Perovskites are also being explored for use in biosensors, where their optical and electronic properties can be leveraged for detecting biological molecules.
Perovskite chemistry is a cornerstone of modern materials science, offering a wealth of opportunities for innovation in technology. The unique properties and structural flexibility of perovskites make them indispensable in the development of next-generation devices for energy, electronics, and environmental applications. As research in this field continues to advance, the potential for new and transformative technologies based on perovskite materials is vast.
Perovskite Formula and Composition
Perovskites are a class of materials with the general formula ABX₃, where:
- A is a larger cation,
- B is a smaller metal cation,
- X is an anion (typically oxygen or a halide).
This structure allows for a wide range of substitutions and modifications, leading to materials with diverse and tunable properties. Below is a detailed explanation of each site in the perovskite structure and its role.
General Perovskite Formula (ABX₃)
The ABX₃ structure consists of:
- A three-dimensional network of corner-sharing BX₆ octahedra (where the B-site cation is surrounded by six X-site anions).
- The A-site cation occupies the interstitial spaces between the octahedra, stabilizing the structure.
This arrangement is highly flexible, allowing for a variety of elements to occupy the A, B, and X sites, which in turn influences the material’s properties.
Explanation of A-Site Cation
The A-site is typically occupied by larger cations, which can be:
- Inorganic cations:
- Cesium (Cs⁺): A common inorganic cation used in perovskites due to its stability and ability to form highly efficient photovoltaic materials.
- Rubidium (Rb⁺): Sometimes used in combination with Cs⁺ to improve stability and performance.
- Organic cations:
- Methylammonium (MA⁺, CH₃NH₃⁺): One of the most widely used organic cations in hybrid organic-inorganic perovskites, particularly in solar cells. It contributes to high efficiency but can be less thermally stable.
- Formamidinium (FA⁺, CH(NH₂)₂⁺): Larger than MA⁺, it offers better thermal stability and a broader absorption spectrum, making it suitable for high-performance solar cells.
Role of the A-site cation:
- Stabilizes the perovskite structure.
- Influences the tolerance factor (a measure of structural stability).
- Modulates the bandgap and optoelectronic properties.
Role of B-Site Metal Cation
The B-site is occupied by smaller metal cations, which are often transition metals or post-transition metals. Common examples include:
- Lead (Pb²⁺):
- The most widely used B-site cation in halide perovskites due to its ability to form highly efficient solar cells with excellent optoelectronic properties.
- However, lead toxicity is a concern, driving research into lead-free alternatives.
- Tin (Sn²⁺):
- A promising lead-free alternative, tin-based perovskites have narrower bandgaps, making them suitable for tandem solar cells.
- However, Sn²⁺ is prone to oxidation, leading to stability issues.
- Titanium (Ti⁴⁺):
- Commonly used in oxide perovskites (e.g., BaTiO₃), which exhibit ferroelectric and piezoelectric properties.
- Not typically used in halide perovskites.
Role of the B-site cation:
- Determines the electronic and optical properties of the material.
- Influences the bandgap and charge carrier mobility.
- Plays a key role in the stability and performance of the material.
Importance of X-Site Anion
The X-site is occupied by anions, which can be:
- Halides (Cl⁻, Br⁻, I⁻):
- Iodide (I⁻): Most commonly used in halide perovskites (e.g., MAPbI₃) due to its ability to form materials with ideal bandgaps for solar cells.
- Bromide (Br⁻): Used to tune the bandgap to higher energies, making it suitable for blue LEDs and tandem solar cells.
- Chloride (Cl⁻): Often used in small amounts to improve crystallinity and stability.
- Oxygen (O²⁻):
- Found in oxide perovskites (e.g., SrTiO₃, LaMnO₃), which are used in applications like catalysis, superconductors, and ferroelectrics.
Role of the X-site anion:
- Determines the crystal structure and stability of the perovskite.
- Influences the bandgap and optoelectronic properties.
- Halides are particularly important in tuning the optical properties of perovskites for solar cells and LEDs.
Tolerance Factor and Stability
The stability and formability of a perovskite structure are often evaluated using the tolerance factor (t), given by:
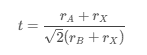
where ( rA ), (rB ), and ( rX ) are the ionic radii of the A, B, and X ions, respectively. For a stable perovskite structure, the tolerance factor should ideally be between 0.8 and 1.0.
Summary of Composition and Roles
| Site | Elements | Role |
|---|---|---|
| A-site | Cs⁺, MA⁺, FA⁺, Rb⁺ | Stabilizes structure, influences bandgap and stability |
| B-site | Pb²⁺, Sn²⁺, Ti⁴⁺ | Determines electronic and optical properties, influences charge transport |
| X-site | Halides (I⁻, Br⁻, Cl⁻), O²⁻ | Tunes bandgap, influences stability and optoelectronic properties |
The ABX₃ formula provides a versatile framework for designing perovskites with tailored properties for specific applications. By carefully selecting the A, B, and X components, researchers can optimize perovskites for use in solar cells, LEDs, sensors, and other advanced technologies. The interplay between these sites is key to unlocking the full potential of perovskite materials in modern science and technology.
Perovskite Structure and Properties
Perovskite Structure and Its Three-Dimensional Crystal Lattice
The perovskite structure is defined by the general formula ABX₃, where:
- A is a larger cation (e.g., Cs⁺, MA⁺, FA⁺),
- B is a smaller metal cation (e.g., Pb²⁺, Sn²⁺),
- X is an anion (e.g., halides like I⁻, Br⁻, Cl⁻, or oxygen).
The structure consists of a three-dimensional network of BX₆ octahedra, where the B-site cation is surrounded by six X-site anions. The A-site cations occupy the interstitial spaces between these octahedra, stabilizing the lattice. This arrangement allows for:
- High structural flexibility,
- Tunable electronic and optical properties,
- Tolerance to defects and substitutions.
How the Structure Affects Electronic and Optical Properties
The perovskite structure directly influences its electronic and optical properties:
- Bandgap Tuning:
- The bandgap can be adjusted by varying the A, B, and X components. For example, replacing I⁻ with Br⁻ increases the bandgap, making the material suitable for blue LEDs or tandem solar cells.
- Charge Carrier Mobility:
- The corner-sharing BX₆ octahedra create pathways for efficient charge transport, making perovskites excellent for optoelectronic applications.
- Absorption and Emission:
- Perovskites exhibit strong light absorption and high photoluminescence quantum yields, making them ideal for solar cells and LEDs.
Stability and Reactivity of Perovskite Materials
Despite their excellent properties, perovskites face challenges related to stability and reactivity:
- Moisture Sensitivity:
- Halide perovskites (e.g., MAPbI₃) are prone to degradation in the presence of moisture.
- Thermal Instability:
- Organic cations like MA⁺ can decompose at elevated temperatures.
- Oxidation:
- B-site cations like Sn²⁺ are susceptible to oxidation, leading to material degradation.
- Light-Induced Degradation:
- Prolonged exposure to light can cause ion migration and phase segregation.
Perovskite Nanomaterials
Introduction to Perovskite Nanocrystals and Their Chemical Behavior
Perovskite nanocrystals are nanoscale versions of perovskites with unique properties:
- Quantum Confinement: At nanoscale dimensions, quantum effects become significant, leading to tunable emission wavelengths and high color purity.
- Surface Chemistry: The large surface-to-volume ratio of nanocrystals makes surface chemistry critical for stability and performance.
Use of Perovskite Quantum Dots in Improving Solar Efficiency
Perovskite quantum dots (QDs) are used in:
- Solar Cells:
- QDs can be incorporated into solar cells to enhance light absorption and charge extraction.
- Tandem Solar Cells:
- QDs with tunable bandgaps are used in multi-junction solar cells to capture a broader range of the solar spectrum.
- Light-Emitting Devices:
- QDs are used in LEDs and displays due to their high color purity and efficiency.
Chemical Aspects in Perovskite Solar Cells
How Perovskite Solar Cell Structure Influences Performance
The structure of a perovskite solar cell typically includes:
- Perovskite Absorber Layer:
- The ABX₃ material absorbs sunlight and generates electron-hole pairs.
- Electron and Hole Transport Layers:
- These layers facilitate the separation and extraction of charge carriers.
- Electrodes:
- Collect and transport charges to the external circuit.
The efficiency of the solar cell depends on:
- The quality of the perovskite layer (e.g., crystallinity, defect density),
- The interfaces between layers,
- The stability of the materials under operational conditions.
Chemical Challenges, Including Stability and Degradation
Key challenges include:
- Moisture and Oxygen Sensitivity:
- Encapsulation techniques are used to protect the perovskite layer.
- Ion Migration:
- Under electric fields or light, ions can migrate, leading to performance degradation.
- Thermal Stability:
- High temperatures can cause decomposition of organic cations.
Advancements in Material Synthesis for Perovskite Solar Panels
Recent advancements include:
- Compositional Engineering:
- Mixing cations (e.g., Cs⁺, MA⁺, FA⁺) and anions (e.g., I⁻, Br⁻) to improve stability and efficiency.
- Additive Engineering:
- Using additives to passivate defects and enhance crystallinity.
- Interface Engineering:
- Optimizing the interfaces between layers to reduce recombination losses.
Mining and Synthesis of Perovskites
Perovskite Mining and Natural vs. Synthetic Production
- Natural Perovskites: Found in mineral deposits (e.g., calcium titanate, CaTiO₃), but these are not typically used in modern applications.
- Synthetic Perovskites: Most perovskites used in technology are synthesized in the lab due to the need for high purity and specific compositions.
Chemical Processes Involved in Making High-Purity Perovskite Materials
- Solution Processing:
- Precursors are dissolved in solvents and deposited as thin films (e.g., spin coating).
- Vapor Deposition:
- Precursors are evaporated and deposited onto substrates under controlled conditions.
- Solid-State Reactions:
- High-temperature reactions between solid precursors to form the perovskite phase.
Conclusion
Summary of Key Chemical Properties
- Perovskites are highly tunable materials with the general formula ABX₃.
- Their structure and composition determine their electronic, optical, and stability properties.
- They are widely used in solar cells, LEDs, and other optoelectronic devices.
Future Research Directions in Perovskites for Solar Cells and Energy Applications
- Stability Improvements:
- Developing more stable compositions and encapsulation techniques.
- Lead-Free Perovskites:
- Exploring non-toxic alternatives like tin-based perovskites.
- Scalable Synthesis:
- Developing cost-effective and scalable manufacturing processes.
- New Applications:
- Expanding the use of perovskites in energy storage, catalysis, and quantum computing.
Perovskite chemistry continues to be a vibrant field of research, with the potential to revolutionize renewable energy and advanced technologies.
